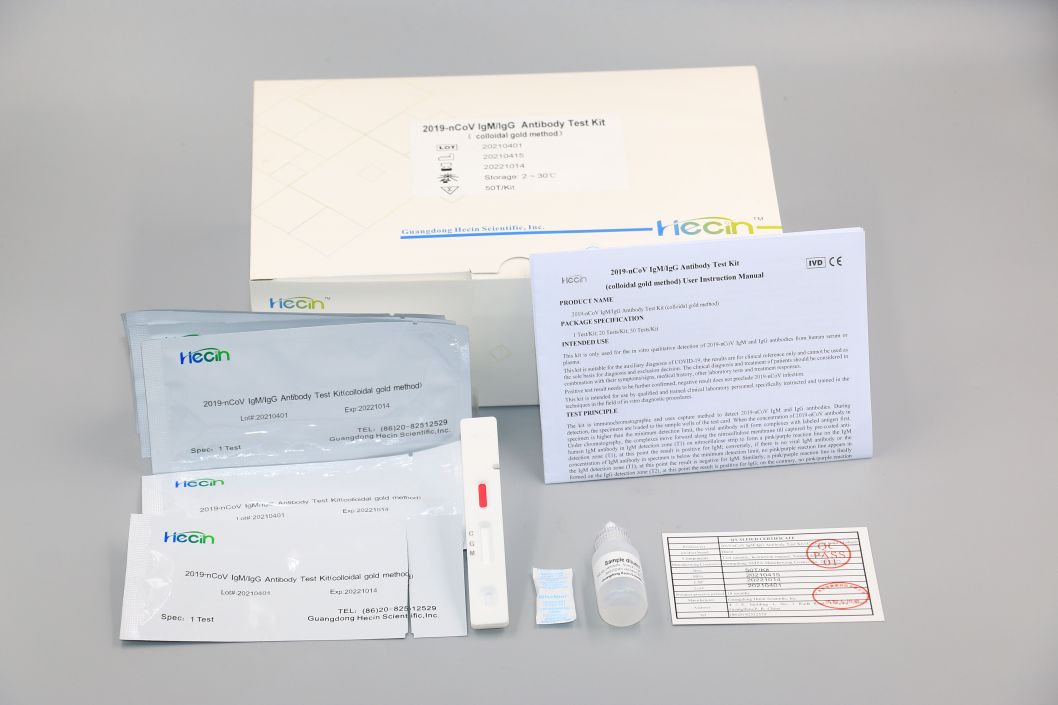
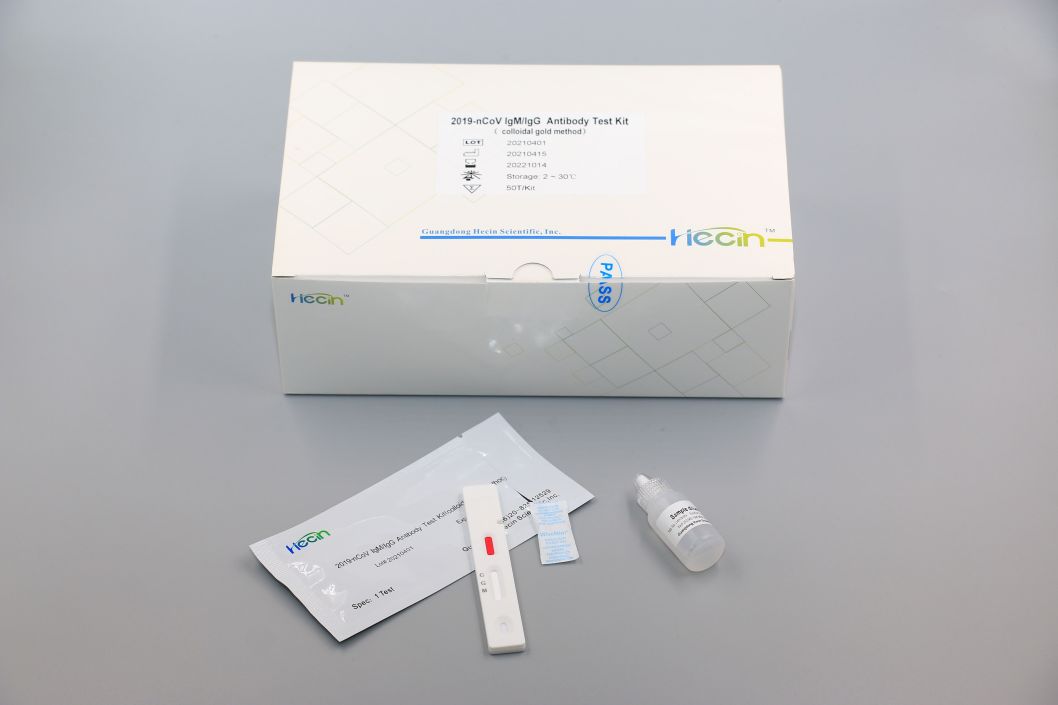
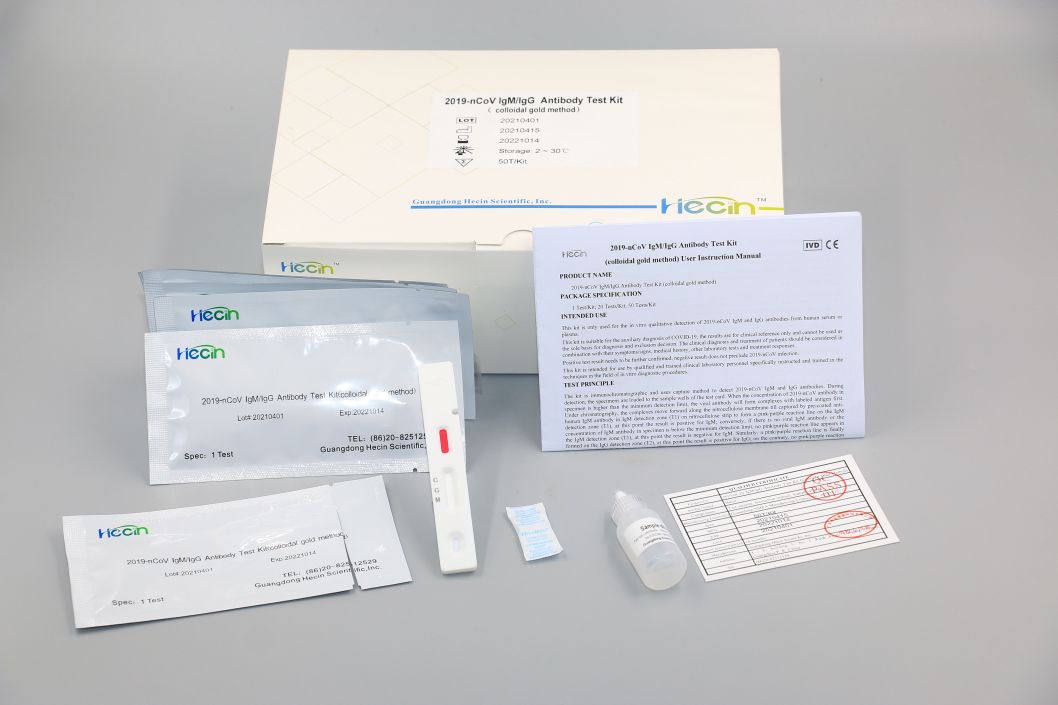
2019-nCoV IgM/IgG Antibody Test Kit (colloidal gold method)
Application scenarios
This kit is suitable for the auxiliary diagnosis of 2019-nCoV (COVID-19).
The clinical diagnosis and treatment of patients should be considered in combination with their symptoms/signs, medical history, other laboratory tests and treatment responses.
Advantages
Advantage
IgM and IgG were in the same card
Detection of two antibodies at the same time, which can indicate the existence of infection or previous infection, improve the detection accuracy.
High specificity
the OD value detected by ELISA was about 0.9~1.1. There was no cross reaction between the kit and 96 pathogens.
Easy Operate
The operation is simple, no instrument detection is needed, the results can be obtained within 15 minutes.
Important auxiliary diagnosis scheme
As an important auxiliary diagnostic scheme of 2019-nCoV (COVID-19) detection, it is suitable for detection after 7 days of symptoms.
Performance
IgG
Sensitivity: 88.68% (76.97~95.73%)
Specificity: 100%(95%CI: 95.85~100%)
Total consistent: 95.71% (95%CI: 90.91~98.41%)
IgM
Sensitivity: 88.17% (79.82~93.94%)
Specificity: 98.34% (95.81~99.55%)
Total consistent: 95.51% (95%CI: 92.70~97.46%)
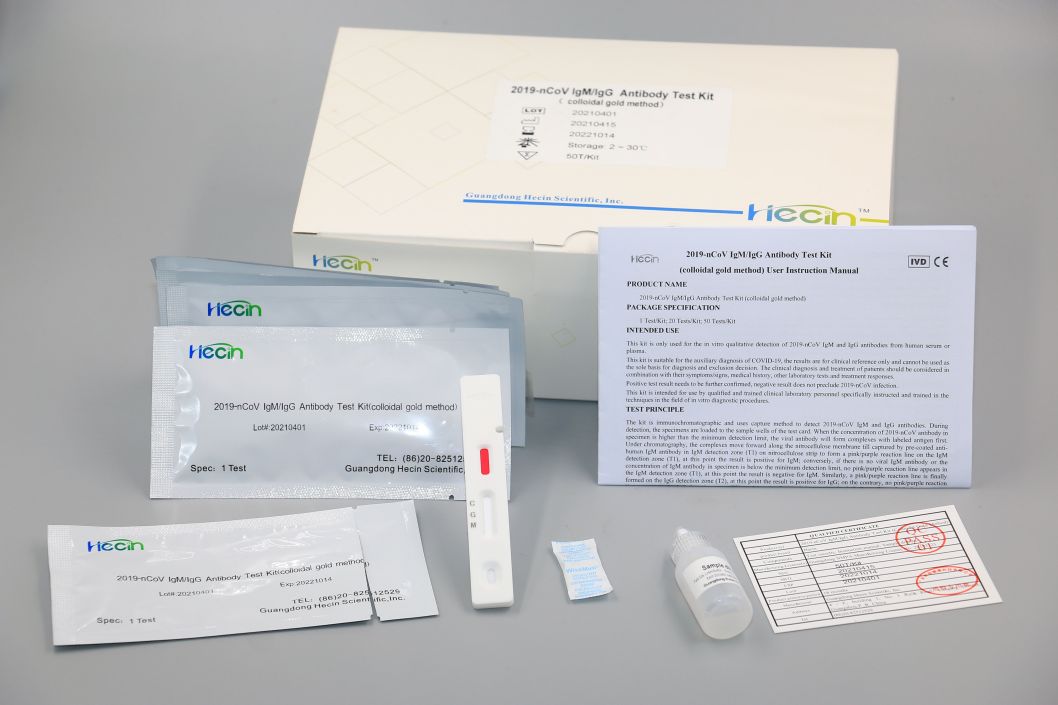
Components
|
Components |
Loading quantity (Specification) |
||
|
1 Test/Kit |
20 Tests/Kit |
50 Tests/Kit |
|
|
Test card |
1 pc |
20 pcs |
50 pcs |
|
Sample diluent |
1 tube (0.2mL) |
1 bottle (2mL/bottle) |
1 bottle (6mL/bottle) |
Test Procedure
1.Blood sample collection.
2.Load 10μL into the sample well of the test card by pipette.
3.Add 2 drops (approximately 80 μL) of sample diluent into the sample well of the test card.
4.read the chromogenic results in the detection zone between 15~20 minutes to ensure proper test performance.
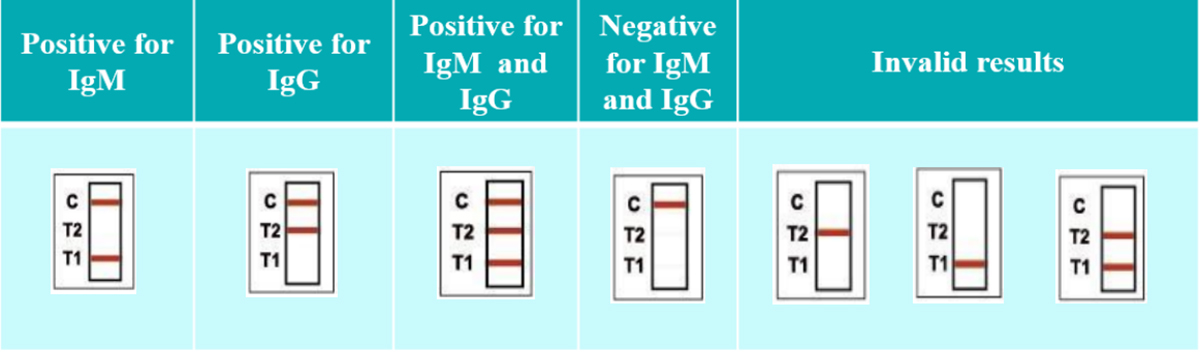
Interpretation of Result
Analytical specificity
This test kit can be used to detect 2019-nCoV, Influenza A virus (2009 H1N1,H1N1,H3N2,H5N1, H7N9), Influenza B virus (Yamagata, Victoria) specifically.



Product specification
IgM/IgG Antibody Test Kit (Colloidal Gold Method)
50T