COVID-19 IgG Antibody Test Kit (colloidal gold method)
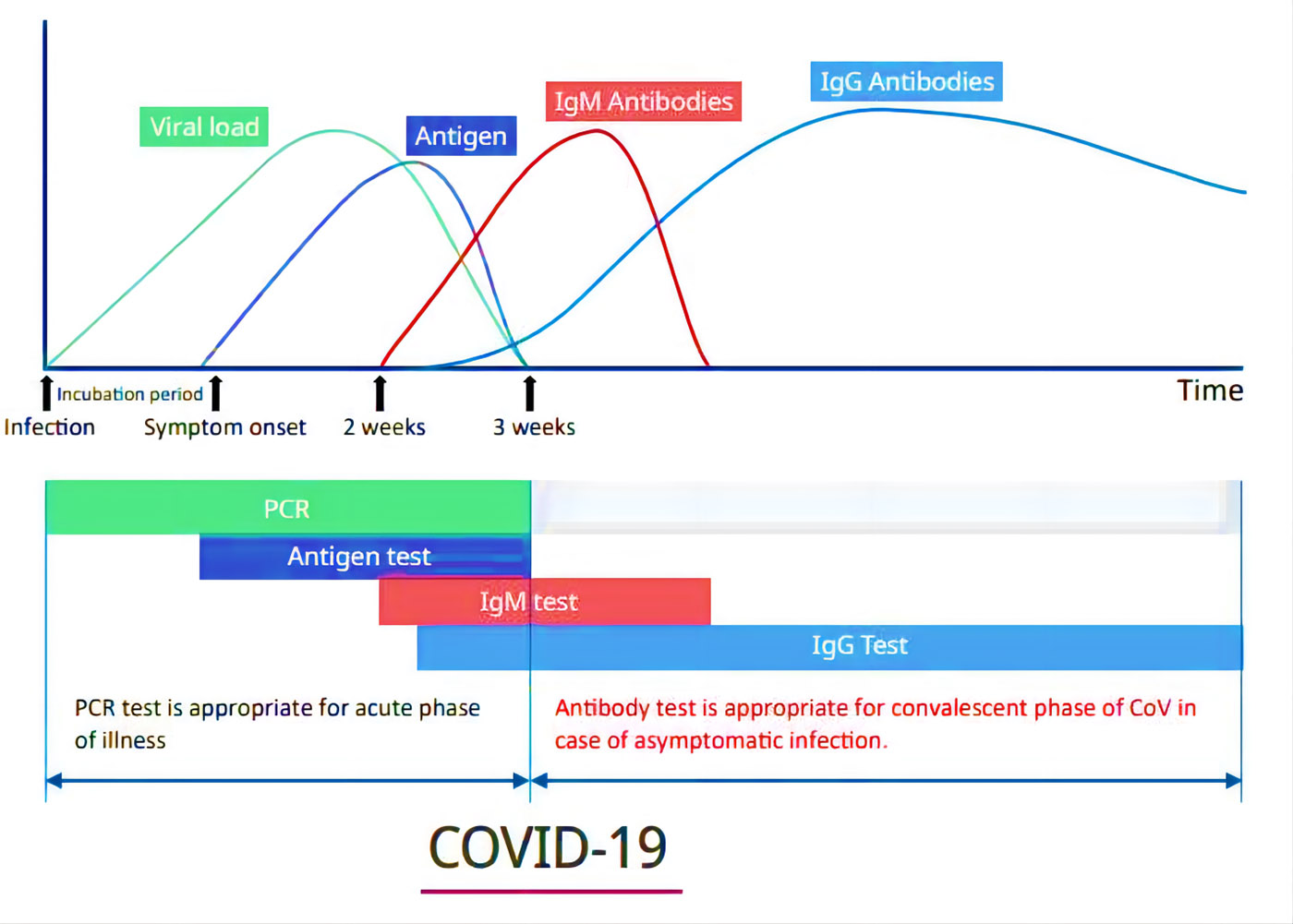
Clinical significance of IgG antibody detection
After stimulated by 2019-nCoV (COVID-19) virus antigen, IgG was differentiated into plasma cells. At the same time, a small number of B cells will differentiate into memory B cells. When the body is exposed to 2019-nCoV (COVID-19) virus antigen again, memory B cells can produce specific IgG antibody rapidly. For early screening, IgM is often used as the test object, for later and retrospective testing, IgG is often used as the test object.
Feature
This product is jointly produced by our company under the guidance of academician Zhong Nanshan and the State Key Laboratory of Respiratory Diseases, with a unique platform advantages.
It is the first company in China that add S protein to the coating antigen. S protein has receptor binding region (RBD) that specifically binds to IgG. It can detect the antibody that has protective effect after infection of 2019-nCoV.
Sensitivity:87.30 % (95%CI: 79.08%~95.52%)
Specificity:100% (95%CI: 99.78%-100%)
Total clinical compliance rate:94.67% (95%CI: 91.07%-98.26%)
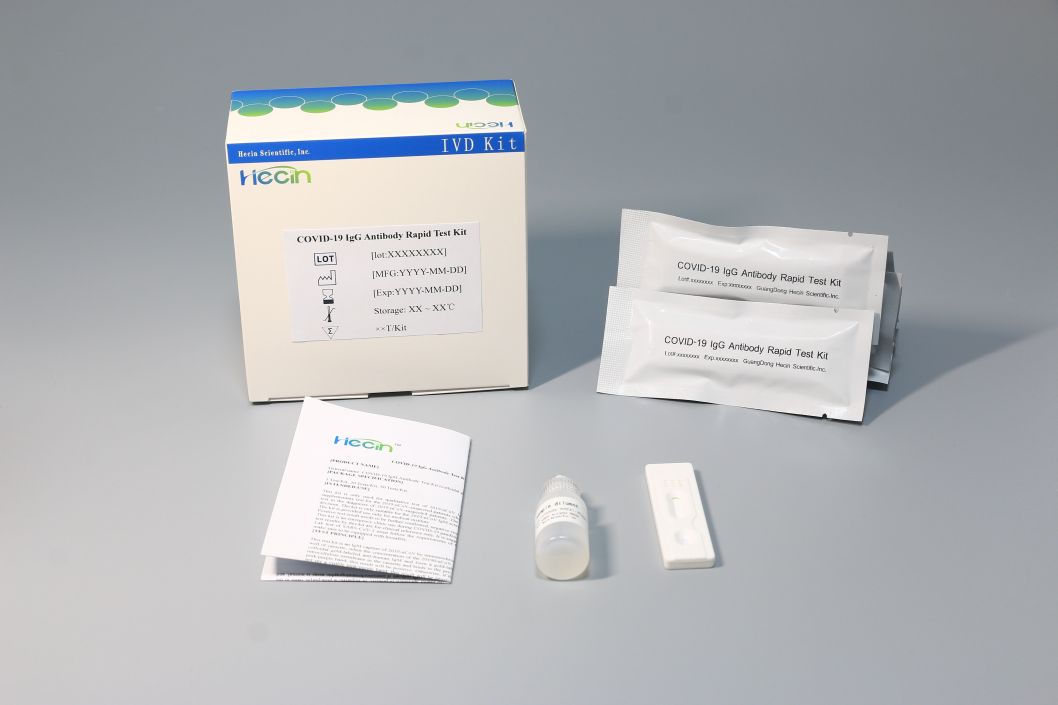
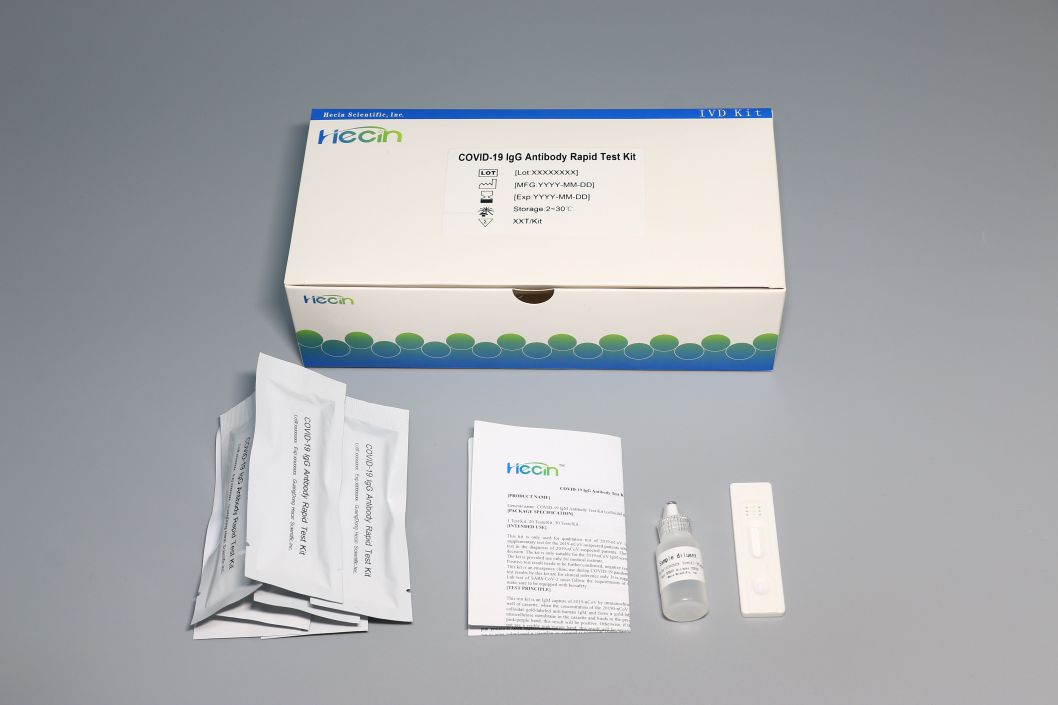
This product is a single card setting, which avoids the risk of non-specific reaction with IgM in the same card slot in technical design. It is easy to operate, without the instrument and the result is given in 15 minutes, which realize the real-time detection.
This product specification has 1 pcs, 20 pcs, 50 pcs, the customers may choose freely according to the demand.
This product can combined with IgM product supplementary detection and use for single follow-up of the prognosis of the general population with fever of unknown cause, which can greatly improve the prevention and control, also effectively track the population that may be infected.
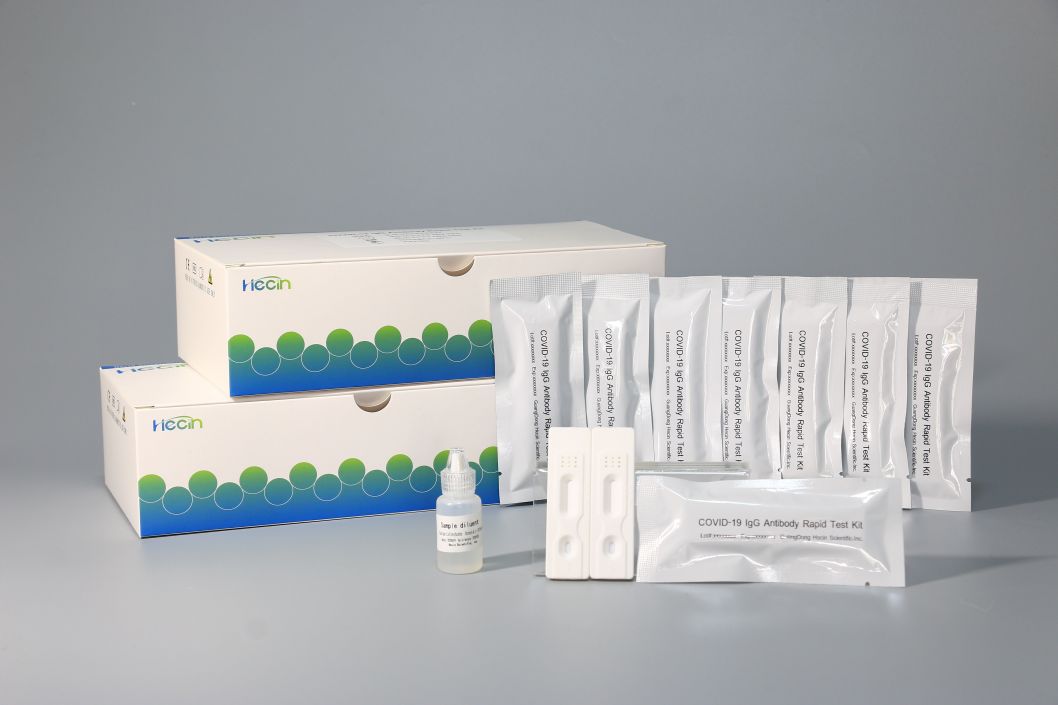
Product Specification Display
IgG Kit (50 PCS)
IgG Kit (20 PCS)
20T
50T