HBoV Nucleic Acid Test Kit (PCR- fluorescence probe method)
HBoV Nucleic Acid Test Kit (PCR- fluorescence probe method)
Introduction
Human Bocavirus infection is mainly manifested as rhinitis, pharyngitis, pneumonia, acute otitis media or gastroenteritis, and symptoms such as cough, dyspnea, chills, fever, nausea, vomiting, and diarrhea may occur. Human Bocavirus is positive in 1% to 10% of respiratory specimens from children and adults with acute respiratory disease.
This kit is intended for qualitative typing detection of human bocavirus nucleic acid in human serum or plasma samples. This kit uses the highly conserved sequence VP gene in the HBoV gene as the target region, and designs specific primers and TaqMan fluorescent probes, and realize the rapid detection and typing of dengue virus through real-time fluorescent PCR.
Performance
Operation steps
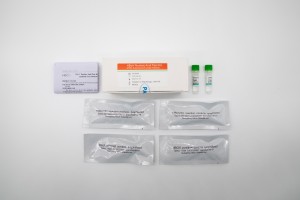
| Components | 48T/Kit | Main Ingredients |
| HBoV/IC reaction mixture, lyophilized | 2 tubes | Primers, probes, PCR reaction buffer, dNTPs, Enzyme, etc. |
| HBoV positive control, lyophilized | 1 tube | Pseudoviral particles including target sequences and internal control sequences |
| Negative control (Purified water) | 3mL | Purified water |
| DNA internal control, lyophilized | 1 tube | Pseudoviral particles including M13 |
| IFU | 1 unit | User Instruction Manual |
-
Hecin Product Profile