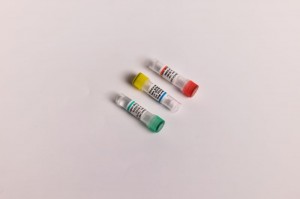
2019-nCoV Nucleic Acid Test Kit (PCR- fluorescence probe method)
Product Advantages
Transport within 37 ℃ for 3 months:Lyophilized reagent is more stable; Transportation conditions: ≤37℃, stable for 3 months;
Pre-filled to save operation steps:The amplification reaction solution was premixed together,Reduce laboratory operations.
“Sample in, result out”:”Rapid PCR Solution support”. It only takes 30 minutes to test on Hecin’s qPCR instrument, but the common testing time is about 110 minutes, can effectively save time.
Applicable to different sample types:Specimen type: nasopharyngeal swab;oropharyngeal swabs; sputum;alveolar lavage fluid specimens.
High sensitivity to improve detection rate:The Limit of Detection (LoD) of 2019-nCoV is 400 copies/mL. Coincidence rate of positive or negative reference: 100%
Advanced freeze-drying process
Hecin experimental verification data show that no difference in performance before and after freeze-drying
Feature
Target: ORF1ab and N genes.
Sample: Human nasopharyngeal swabs, oropharyngeal swabs, sputum, alveolar lavage fluid specimens.
Specification: Single tube for single test; 12×8T/Kit; Large package: 4×24T/Kit;
Official agency recognition: Obtained EU CE certification, product quality is reliable
Performance
Limit of Detection: 400 copies/mL
Recommended scheme
Scheme 1. Specimen release agent + 2019-nCoV Nucleic Acid Test Kit (PCR- fluorescence probe method) (8*12T) + UltraFast QPCR (HC800).
Scheme 2. Specimen Collection Medium or Specimen release agent + Nucleic Acid Purification Kit + 2019-nCoV Nucleic Acid Test Kit (PCR- fluorescence probe method) (4*24T) + Regular Real-Time PCR systems( Ex: ABI7500)
Registration Certificate



HS27- 12X8T/Kit
HS27- 4X24T/Kit