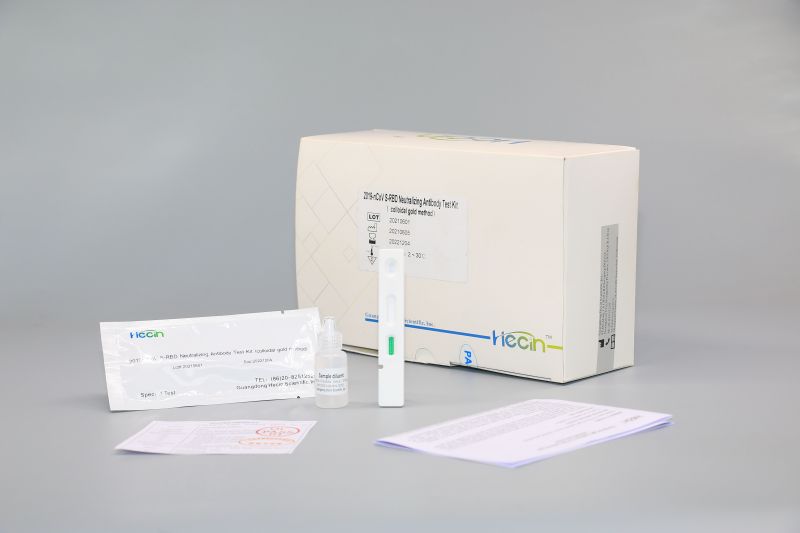
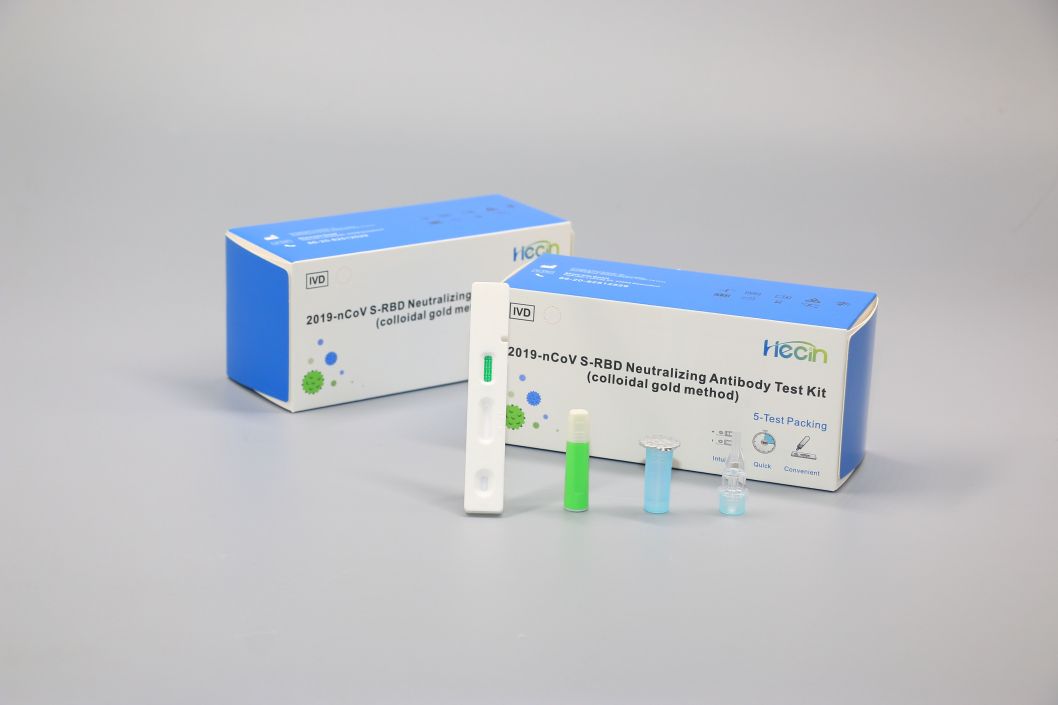
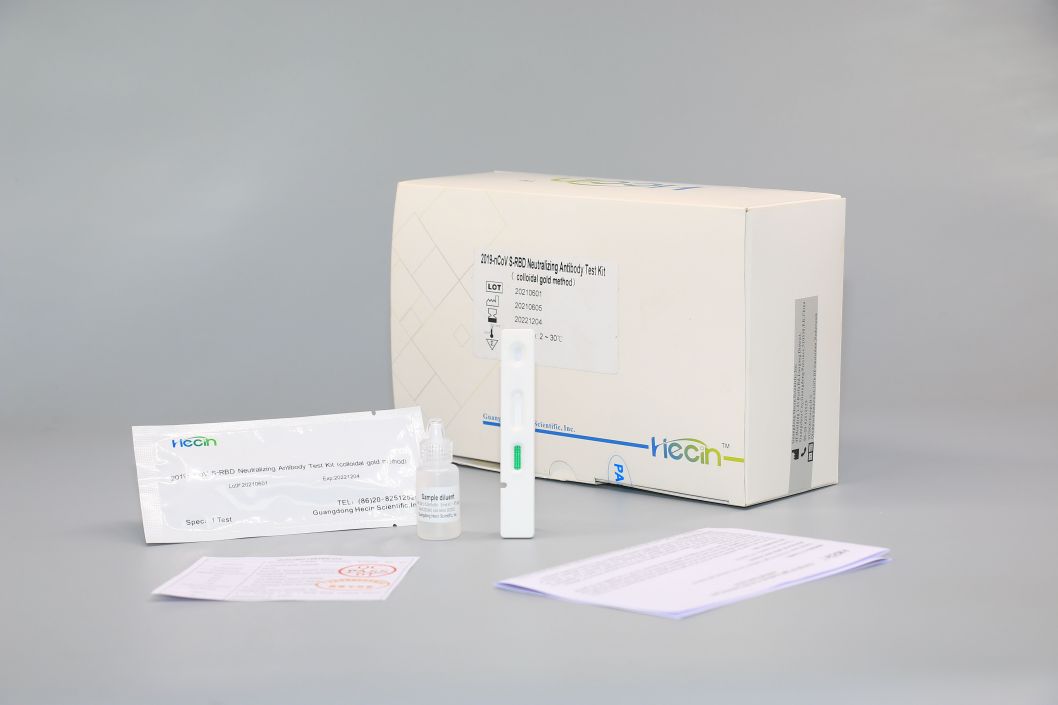
2019-nCoV S-RBD Neutralizing Antibody Test Kit (colloidal gold method)
Introduction
At present, all candidate vaccines of 2019-nCoV in clinical development are administered by intramuscular injection. Intramuscular or intradermal vaccination can lead to strong induction of serum IgG
More than 180 vaccine candidates, based on several different platforms are currently in development against 2019-nCoV.
The S protein is the major target of neutralizing antibodies;
Many of these neutralizing antibodies target the RBD of S protein.
How to judge the efficiency of 2019-nCoV vaccine?--- Neutralizing Antibody Test Kit
Advantage
Pre vaccine testing
Before vaccination, candidates can detect the neutralizing antibody of RBD to determine whether vaccination is necessary;
Most vaccines are covered
It can detect neutralizing antibodies produced by most vaccines on the market;
Fast and convenient
The operation is simple, no instrument detection is needed, the results can be obtained within 15 minutes.
Identification function
It can distinguish the neutralizing antibody of 2019-nCoV produced by 2019-nCoV vaccine or the antibody produced by 2019-nCoV infection for a certain kind of vaccines, such as Viral vector (Non-replicating) vaccine, RNA base vaccine and Protein subunit vaccine;
Whole blood test
Whole blood test makes the operation more convenient;
Scope of application
Pre vaccination
Determine whether they have been infected with new coronavirus and whether they still need to be vaccinated;
Vaccination period
Determine whether effective new neutralizing antibody is produced;
Late stage of inoculation
According to the epidemic area of 2019-nCoV, it is suggested to detect the existence of 2019-nCoV neutralizing antibody regularly every three months.
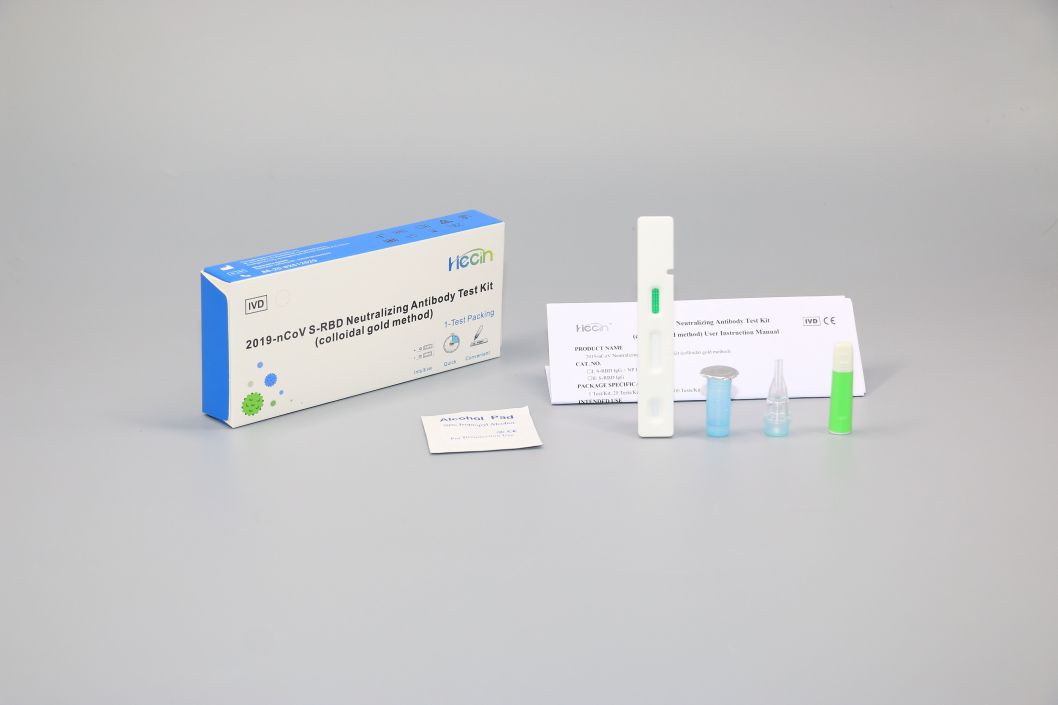
Components
| Components | Main Ingredients | Loading quantity (Specification) | ||
| 1 T/Kit | 20 T/Kit | 50 T/Kit | ||
| Test card | Test strip containing colloidal gold labeled anti-human IgG antibody, colloidal gold labeled anti-chicken IgY antibody, 2019-nCoV S-RBD recombinant protein, Chicken IgY antibody | 1 pc | 20 pcs | 50 pcs |
| Sample diluent | 0.01M Phosphate buffer solution, 0.5% Tween-20 | 0.5mL | 5mL | 10mL |
Performance
| Hecin reagent | Clinical serum virus neutralization test | Total | |
| Positive | Negative | ||
| Positive | Positive | 84 | 9 |
| Negative | Negative | 8 | 198 |
| Total | Total | 92 | 207 |
| Clinical sensitivity | Clinical sensitivity | 84/92 91.30% (95%CI: 83.58%~96.17%) | |
| Clinical specificity | Clinical specificity | 198/207 95.65% (95%CI: 91.91%~97.99%) | |
| Accuracy | Accuracy | 282/299 94.31% (95%CI: 91.05%~96.65%) | |
Hecin reagent Performance against Comparator Method on serum/plasma specimens.
| Hecin reagent | Clinical serum virus neutralization test | Total | |
| Positive | Negative | ||
| Positive | 84 | 8 | 92 |
| Negative | 8 | 199 | 207 |
| Total | 92 | 207 | 299 |
| Clinical sensitivity | 84/92 91.30% (95%CI: 83.58%~96.17%) | ||
| Clinical specificity | 199/207 96.14% (95%CI: 92.53%~98.32%) | ||
| Accuracy | 283/299 94.65% (95%CI: 91.46%~96.91%) | ||
Hecin reagent Performance against Comparator Method on whole blood specimens.
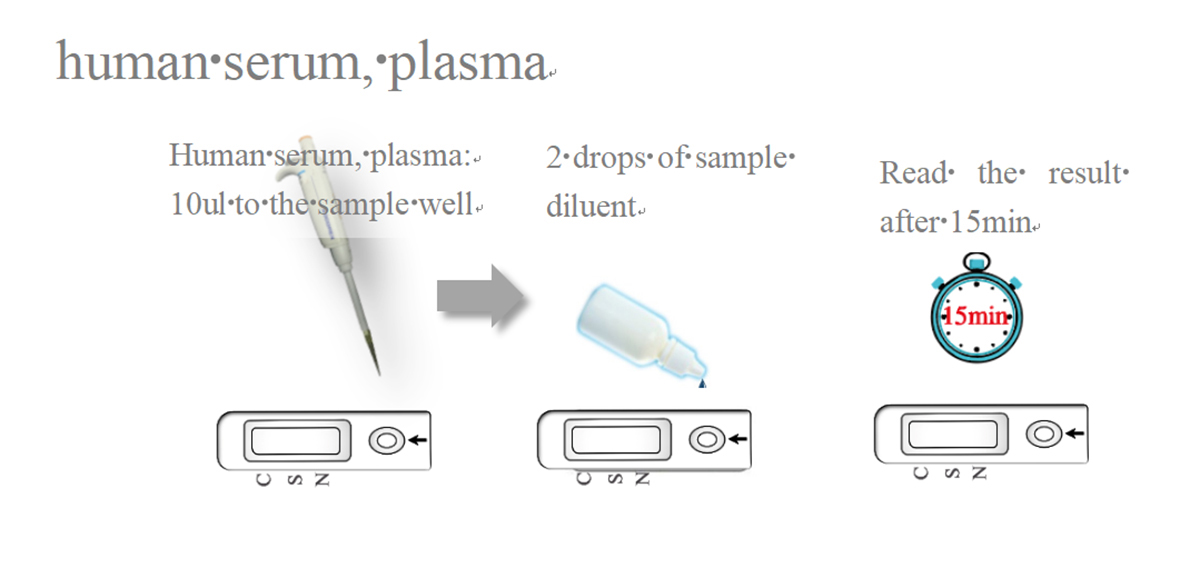
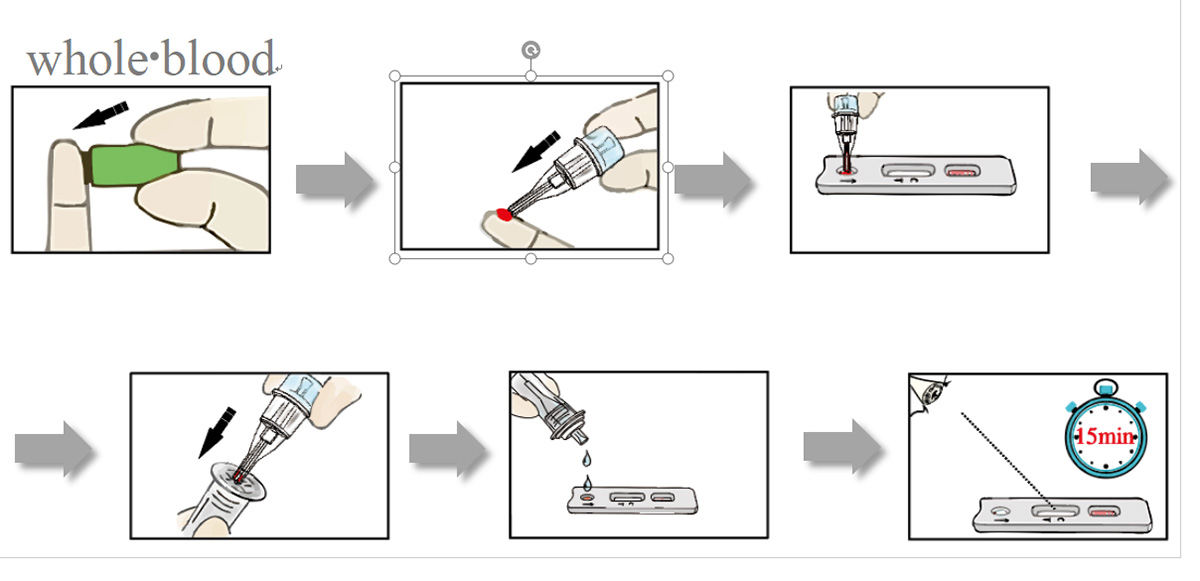
Test Procedure
Registration Certificate


JT08- 1T
JT08- 5T
JT08- 50T